Navigating the Nuance: Education, #Ads, and Therapeutic Goods
If you are a nurse in Australia, you should know the rules about advertising therapeutic goods or making health claims for unregistered products. This is relevant for any health professional who uses social media to promote their services or communicate with the public.
The Therapeutic Goods Association (TGA) defines therapeutic goods as products that:
preventing, diagnosing, treating or easing a disease, condition, defect or injury
affecting, slowing down or changing a bodily function
checking the risk of getting a disease or condition
affecting, regulating or stopping pregnancy (TGA, 2020)
For example, a preganancy test, over the counter pain relief, or a TENS machine would all be classed as therapeutic goods.
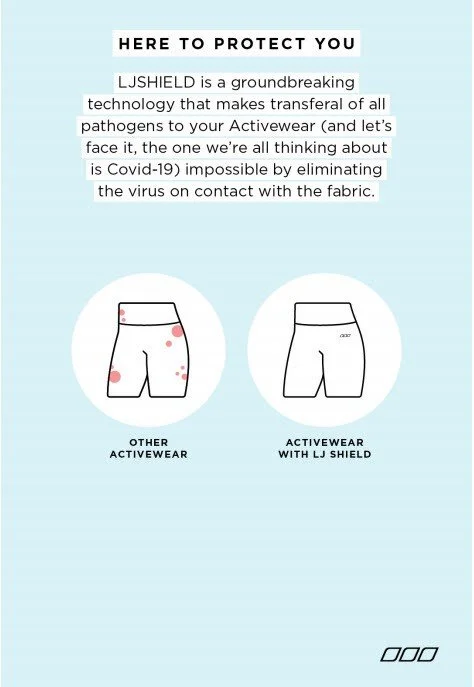
Over the past few years, the TGA has turned their attention to health content online, specifically, social media and therapeutic claims. In July 2022, the TGA updated their Code of Advertising to provide greater clarity on actions that can lead to criminal and civil penalties. The main goal of the Code is to make sure that advertising material is “honest, fair and not misleading.” For example, in 2020 Lorna Jane was found to have made false and misleading statements regarding COVID-19 and their new product “LJSHIELD” (pictured below).
(Source: ACCC)
Why was it misleading? 1. Lorna Jane made therapeutic claims for a product not registered with the TGA. 2. COVID-19 is a restricted representation (you can’t make claims about it!)
So what does this mean for health professionals?
Health professionals are not permitted to endorse (both implicit and explicit endorsement) or provide testimonials for therapeutic goods. The 2022 Code clarified that even retired or no longer registered health professionals may not endorse or provide a testimonial for therapeutic goods.
Ok, but what does endorsement mean?
When the TGA uses the term endorsement, they mean any content or conversation that provides explicit or implicit recommendation of a good or brand. So, let’s use the example of a TGA registered essential oil. Let’s consider Jack – he’s a Registered Nurse and is a network marketing representative (like selling Tupperware) for a popular essential oil company. Jack has a social media profile and uses that platform to advertise his products and talk about his own experiences with the products. Some of the products are therapeutic goods (they have an “L” number). This is in breach of the TGA Code of Advertising in two ways:
Health practitioners are prohibited from endorsing or providing testimonials for TGA registered goods.
Any individual involved with the company (including network marketing) is prohibited from providing testimonials or endorsements related to the product.
Example
In this example, Jack would be in breach of the code of advertising. Specifically:
Jack provides a testimonial
Jack appears to endorse the product by giving it a “thumbs up” and by presenting the product and brand in the imagery
Jack clearly identifies that he is a nurse and therefore cannot provide a testimonial or endorsement of a TGA registered product
There is no educational aspect to this content
Endorsement is not always as explicit as this example, however. Endorsement may also be inadvertent, like sharing a story on Instagram with your own personal medications in the background (see more here). Likewise, even if your intention isn’t to sell a product – maybe you just really believe in it and want to share it with your networks – this could still be in breach of the Code. The reason for this is that nurses (and other health professionals) are highly trusted by the community, and their endorsement holds more weight because of this level of trust. This places the public and community at risk.
Case Study: Dr Valerie Cole
Please note that therapeutic relationships with clients/patients are not always considered at risk of advertisement, but can be depending on the clinicians relationship to the product. That is, if they financially benefit from the recommendation. For example, in 2019 Dr Valerie Cole was reprimanded for recommending a product to patients she sold through a network marketing company, USANA. Of particular note was that Dr Cole also recruited these patients into her sales team and failed to adequately disclose her conflicts of interest. Dr Cole received restrictions on her registration for 12 months.
But what about vaccines?
I’ve seen nurses share about getting their own vaccinations online? Isn’t that a breach? Not necessarily. There are exemptions that exist in the interest of public health initiatives. For example, during the COVID-19 pandemic, an exemption was made to allow health professionals to share content and advertisements regarding the COVID-19 vaccines, provided that the content adhered to a set of principles (you can read that here). Beyond this, there is a distinction between advertisements and educational content. According to the TGA:
“while a disease education activity may make reference to a range of treatment options, if the information provided is likely to encourage consumers to seek to obtain a particular good, or seek a prescription for a particular medicine, then it will be considered an advertisement.” (TGA, 2020)
At this point, most people scratch their heads in confusion. And for good reason, too. The TGA is a governance organisation, which means that the majority of information is policy-focussed, and less communication-focussed. However, they have shown increased interest in educational explainers for health professionals and organisations. I see that as a positive step in the right direction.
So…what does this mean? Is it an ad?
My interpretation of these guidelines is that if:
the information is consistent with clinical guidelines, evidence and/or campaigns, and;
there is clearly a focus on awareness of how the product works (as opposed to a sales pitch), and;
there is no personal financial benefit, and;
the information presented is balanced (risks outlined, no “overstating” language), and;
it does not promote one brand over another,
it would still be judged as educational.
Why do I think this? I have yet to see any cases published describing a penalty for providing educational content that references a therapeutic good. Even in the case of Valerie Cole (above), the issue was that there was an undisclosed conflict of interest. When considering the dilemma of advertisement vs education, the TGA often talks about “reasonableness” - what would a reasonable person think when seeing your content? Would a reasonable person think that your educational content about child health and different classes of antibiotics is an advertisement? Unlikely. Would a reasonable person think your post educating about sunscreen benefits with a discount code to a particular brand think it’s an advertisement? Most likely. You can read more about endorsements with examples here.
In short, you must be aware of regulations governing the advertising of therapeutic goods and making health claims for unregistered products, especially when using social media. However - you don’t need to be afraid of the regulations! There are plenty of ways to provide educational content regarding therapeutics, which are necessary for professional development and public safety. A crucial distinction is made between advertisements and educational content to maintain ethical practices in public health communication. Before you talk about therapeutic goods online, be sure you are within the accepted boundaries of practice.
Still murky? Drop me a line via @j_stokesparish
Want to hear more about this?
Book me for a workshop here.
Disclaimer: This information is educational by nature. If you are unsure of the legal obligations that apply to your own personal situation, please seek legal advice, or contact the TGA.
References:
ACCC, 2021. Lorna Jane pays $5 million over false 'anti-virus activewear' claims. https://www.accc.gov.au/media-release/lorna-jane-pays-5-million-over-false-anti-virus-activewear-claims
Medical Board of Australia, 2019. Queensland GP reprimanded after failing to disclose a conflict of interest. https://www.medicalboard.gov.au/News/2019-06-18-Qld-GP-reprimanded.aspx
TGA, 2020. Advertising guidance for providers of disease education activities. https://www.tga.gov.au/resources/resource/guidance/advertising-guidance-providers-disease-education-activities#:~:text=While%20a%20disease%20education%20activity,will%20be%20considered%20an%20advertisement.
TGA, 2020. Communicating about covid-19. https://www.tga.gov.au/resources/resource/guidance/communicating-about-covid-19-vaccines
TGA, 2020. What are therapeutic goods? https://www.tga.gov.au/about-tga/what-we-do/what-are-therapeutic-goods#:~:text=Therapeutic%20goods%20are%20broadly%20defined,to%20a%20disease%20or%20ailment
TGA, 2023. Activities that represent advertising. https://www.tga.gov.au/resources/resource/guidance/australian-regulatory-guidelines-advertising-therapeutic-goods-argatg/activities-represent-advertising